YOU ARE
A company active in the healthcare sector, a clinical center/investigator, a health league/NGO...
...willing to:
...willing to:
- Conceive a clinical study that is compliant with ethical and regulatory requirements
- Prepare the documentation to obtain the authorisation to run the study
WE OFFER YOU
|
Consulting
Medical writing
|
Please download our flyer.
OUR APPROACH
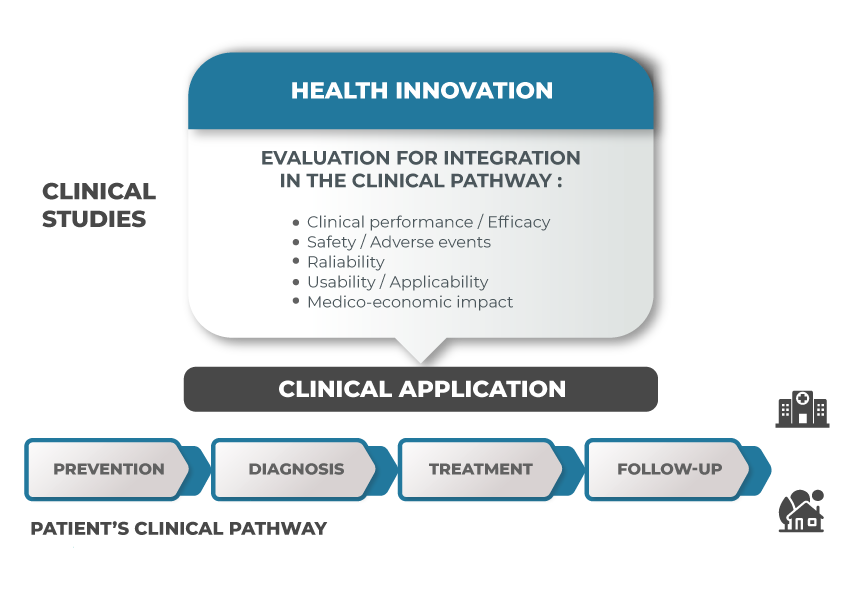
You can outsource individual tasks or the overall study conception and submission to us. Vivactis helps you plan your study from the design to the reporting phase, in order to be compliant with regulatory and ethical requirements.
For CE marking submissions or medico-economic analyses for reimbursement strategies, we collaborate with experts from the field, either within the Vivactis Group or outsourced.
For more information, please contact us !
For CE marking submissions or medico-economic analyses for reimbursement strategies, we collaborate with experts from the field, either within the Vivactis Group or outsourced.
For more information, please contact us !
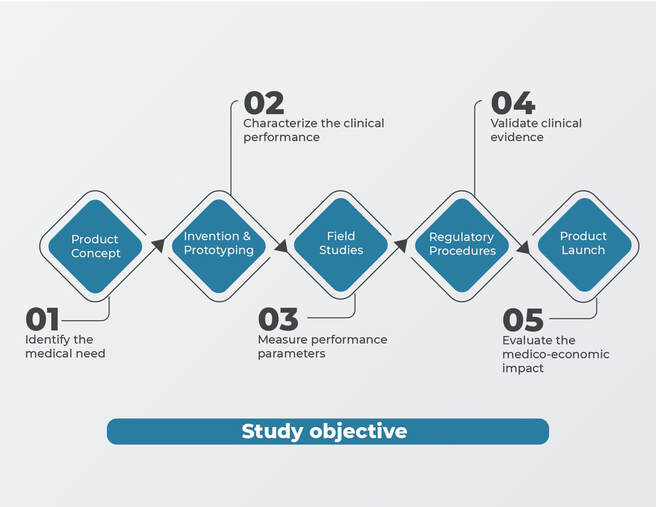
BUSINESS CASES – Conception & Authorisation of Clinical Studies
Context : A Swiss health league wants to run a clinical study in a public hospital to prove the reliability and applicability of a wireless-connected medical device for the monitoring of patients affected by a chronic disease.
Vivactis solution & deliverables :
- Study design (definition of study scope, objectives, outcomes, population…)
- Principal investigator identification
- Edition of the study synopsis
- Edition of the authorisation file for the Ethics Committee (study protocol, informed consent form, case report form, patients & HCPs questionnaires)
- Study registration on clinicaltrials.gov
- Electronic study file submission through the Ethics Committee portal.
Context : A Medtech company wants to investigate the usability for the general practitioners of their new medical device for screening purposes.
Vivactis solution & deliverables :
- Study design
- Physicians identification and recruitment
- Authorisation file submission to the Ethics committee
- Physicians training on the use of the device (development of the training material)
- Organization of a campaign to recruit patients
- Scientific publication of the study results.